Effects of Heat: Change in Physical State
Effects of Heat: Change in Physical State: Overview
This topic covers concepts such as Latent Heat, Latent Heat of Fusion, Latent Heat of Vaporisation, Fusion of Substances, Vaporisation of Substances and Temperature Versus Heat Graph for Heating of Ice.
Important Questions on Effects of Heat: Change in Physical State
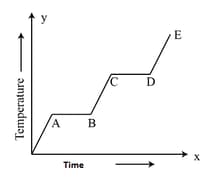
An ice cube is heated and the variation of its temperature with time is shown. The process representing the conversion of water into steam is
How much heat is required to convert of ice at to water at ? (Given specific heat of ice , specific heat of water , Latent heat of fusion )
of steam at is passed into a large block of ice at the mass of ice that melts is
Water and ice having equal masses are placed inside an insulated container. The initial temperatures of water and ice are and respectively. The equilibrium temperature of the system in degrees Celsius is close to (Note: take the specific heat of water ; latent heat of fusion )
Evaporation is the process of changing liquid into vapor,
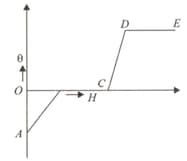
Heat given (H) to a substance was plotted against rise in temperature (0). Which of the following parts of the graph, most correctly
depicts the latent heat of the substance?
Determine the amount of heat required to completely melt of ice (in ) given that latent heat of fusion for ice is .
Distinguish latent heat of fusion from the latent heat of vaporization during state change of substance with graph.
Ice melts when energy in the form of ____ is supplied
Heat is supplied to a solid to raise its temperature across its melting point and boiling point Which of the following graphs correctly represents the relation between heat supplied and temperature ?
A block of ice at is slowly heated and converted to steam at . Which of the following curves represent the phenomenon qualitatively?
Observe the following temperature Vs. time graph and fill in the blank:
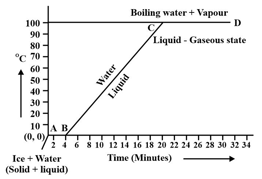
During transition of solid phase to liquid, the object absorbs _____ (electric \chemical \heat) energy, but its temperature does not increase.
Observe the following temperature Versus time graph and fill in the blank.
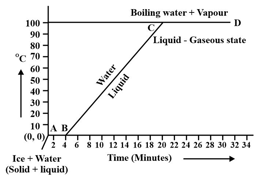
The constant temperature, at which the ice converts into water is called the _____ point of ice.
(Choose from: boiling/melting)
Observe the following temperature Versus time graph and fill in the blank.
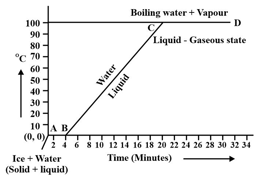
When ice is heated it melts at _____ and converts into water at this constant temperature.
(Choose from: /)
Liquid and solid states of a substance co-exist in thermal equilibrium during the process of:
During the process of fusion:
The specific latent heat of vaporisation for water is large.
Conversion of liquid into vapours is called _____.
The correct reason behind cooling of a room after the water has been sprinkled is
When sun rays are focused on an ice block using a lens of diameter , of ice got melted in . Calculate the heat received per minute per square centimetre. (Latent heat of ice )